
Introduction
ARGEN™ is a versatile instrument for rapid assessment of the stability and viability of therapeutic biopolymers and biopolymer complexes. This technical note captures the value of using ARGEN to determine the thermal and chemical stability of monoclonal antibody (mAb).
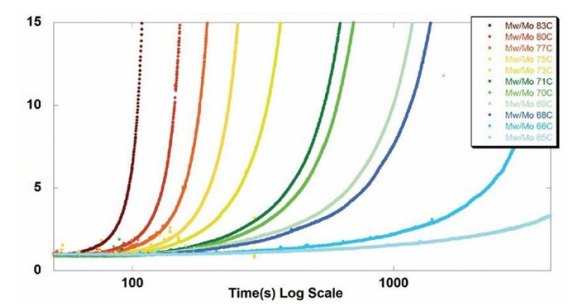
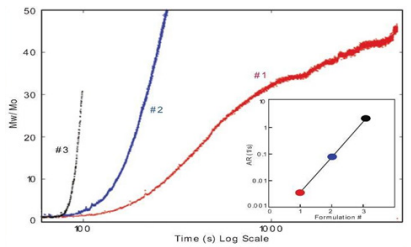
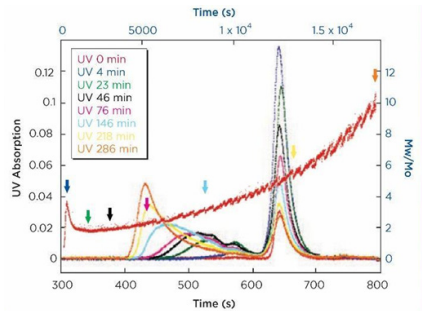
ARGEN™ is a high throughput tool for the rapid assessment of the stability and viability of therapeutic proteins, peptides, and biopolymers. The instrument uses a multi-stressor testing platform powered by static light scattering detection and intuitive data processing. These features enable teams to develop biologic formulations up to 16-fold faster. ARGEN™ utilizes fixed angle (90°), simultaneous multiple sample light scattering (SMSLS) technology. This provides rapid, real-time, continuous data collection for characterizing qualitative and quantitative properties of target molecules. The device is equipped with 16 independently controlled sample cells, permitting the user to establish thermal, chemical, and mechanical (stirring) stress parameters on each sample concurrently. This allows for a highly flexible approach to experimental design. The ARGEN™ control software features an intuitive interface for all aspects of experimental design and independent control of each cell for parallel parameter adjustment and real-time data processing. These experiments evaluate real-time molecular weight changes of a monoclonal antibody across a temperature spectrum. Below, (Figure 1) illustrates the unfolding and subsequent aggregation versus time of a mAb under isothermal regimes with a temperature range of 65°C – 83°C. Each curve (11 total) represents the relative molecular weight (Mw(t)/Mo) of the antibody versus time under an isothermal condition. Mw(t)/Mo is the normalized ratio of the molecular weight with respect to the initial, non-aggregated sample mass. Mo is the molecular weight of the native, unaggregated protein, and Mw(t) is the weight average molecular weight of all native or aggregated species at a time point (t). Changes in the Mw(t) represent proportional changes in the aggregate mass and concentration. This output is used to derive the aggregation rate (AR). These experiments clearly allow for the characterization of the thermostability for the monoclonal antibody. (Figure 2) illustrates the effects of pH on the stability of a monoclonal antibody. Under identical temperature and mechanical stress conditions, changes in pH significantly perturbed stability with varying aggregation rates, as indicated by the change in Mw(t)/Mo versus time. Interestingly, the aggregation rates were different under each condition, providing pertinent insights into ideal storage and purification conditions. Performing experiments with ARGEN under various solution conditions can provide valuable data for ab initio development and during downstream production and processing. Data collected with ARGEN allows users to rapidly vet the quality and viability of candidates. Mw/Mo output quickly permits the identification of abnormal aggregation rates and molecular weight changes, saving time and resources required for orthogonal technique verification. Once a viable candidate is identified using ARGEN, this data can be reconciled with SEC experiments or similar techniques. This approach can lead to an expedited understanding of ideal formulation development conditions. (Figure 3) illustrates the super imposition of the SEC elution profile and variations in Mw/Mo ratios (from ARGEN) of a monoclonal antibody over time. ARGEN reveals the aggregation rates and integrated changes in oligomerization states versus time. Conversely, SEC shows only discrete transitions from monomer to higher order oligomers. Coupling data from these techniques provides robust evidence for the identification of biopolymer stability. In these experiments, ARGEN was used to characterize the thermal and chemical stability of a monoclonal antibody and subsequently establish quantitative and qualitative properties such as pH and temperature dependence on aggregation kinetics. Additionally, SEC elution profiles were superimposed onto Mw/Mo output, providing a robust comparison and identification of the thermal and chemical stability landscape for the mAb. These studies demonstrate ARGEN’s power and capability to provide pertinent stability data necessary to expedite the development of therapeutic biopolymers. ARGEN™: Smart & Rapid Therapeutic Biopolymer Development
How ARGEN™ Works
ARGEN™ Intuitive Control Software
Thermal and Chemical Stability
Probing the Thermal and Chemical Stability of a Monoclonal Antibody
Effects of pH on Monoclonal Antibody Stability
Coupling ARGEN Data with Size Exclusion Chromatography (SEC) to Monitor Thermal and Chemical Stability
Conclusion

