Why this matters
Polymethylmethacrylate (PMMA) is a highly transparent and weatherable polymer that is used as a lighter and more impact resistant alternative to glass. PMMA is used in various applications, including shatter-resistant panels for windows, bathtubs, LCD screens, coatings, and various medical and dental applications.
Performance in these applications depends on bulk properties of the polymeric material, including strength, brittleness, glass transition temperature and solubility. These bulk properties are largely determined by the intrinsic properties of the individual polymer molecules, such as intrinsic viscosity, molecular weight and molecular weight distribution. Monitoring polymer properties during synthesis is the first step toward achieving control of the production process, as well as designing materials to customer specifications.
ACOMP is an online, smart manufacturing system that continuously monitors polymer properties in real time. ACOMP uses a well-known, non-chromatographic technique to measure monomer and polymer concentration, intrinsic viscosity and weight average molecular weight. By applying ACOMP’s capabilities to the polymerization of polymethylmethacrylate, the properties of the polymer can be monitored and controlled.
Using ACOMP to Monitor the Synthesis of Polymethylmethacrylate
The polymerization of polymethylmethacrylate can occur through various methods, including in liquid-phase solution, in a bulk extrusion process, and via casting. Crosslinking PMMA by the addition of a compound such as ethylene glycol dimethacrylate (EGDMA) yields a high molecular weight, more ductile polymer, which has a higher glass transition temperature (Tg) that is desirable for certain applications. In this technical note we will illustrate how ACOMP can successfully monitor and characterize the solution polymerization of PMMA in the presence and absence of this crosslinker.
Methodology
The ACOMP system uses an ultraviolet (UV) absorption detector and a differential refractometer (DRI) for measuring the concentrations of monomers and polymer. The UV detector can measure absorption at up to four wavelengths, 240 nm, 245 nm, 250 nm and 255 nm, depending on the need in the specific polymerization system. A single capillary viscometer and 5-angle static light scattering detector were used for measuring intrinsic viscosity and weight-average molecular weight, respectively. In these experiments, ACOMP extracted a sample from a reactor recirculation loop at the rate of 0.5 ml/min. The reactor sample was automatically and continuously diluted 55 times in butyl acetate (BA), the same solvent used in the reaction.
To illustrate the utility of ACOMP to monitor and characterize the effect of EGDMA as a crosslinker, two batch solution polymerizations of MMA were conducted under identical conditions. The reactor was charged with 30% by weight of total monomer in butyl acetate and, after allowing the signals from ACOMP to stabilize, the reaction was initiated with 3.5% azobisisobutyronitrile (AIBN) at 70˚C. The first reaction was conducted without crosslinker, whereas the second reaction was carried out with the addition of 0.1% EGDMA, as shown in Table 1.
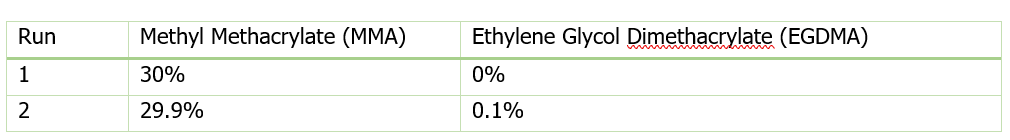
Figure 1 shows data from Run 2, the reaction with crosslinker. In this reaction ACOMP successfully monitors the evolution of polymer properties in real time, after the reaction was initiated at around 5000 seconds. The UV at 255 nm, depicted in blue, decreases exponentially as the double bonds in the monomer polymerize. The DRI signal (red) increases with the increase of polymer concentration since the refractive index of the polymer is higher than that of the monomer. The viscometer pressure (black) and light scattering intensity (green) increase due to both the continuously increasing molecular weight of the polymer chains and the increasing concentration of polymer.
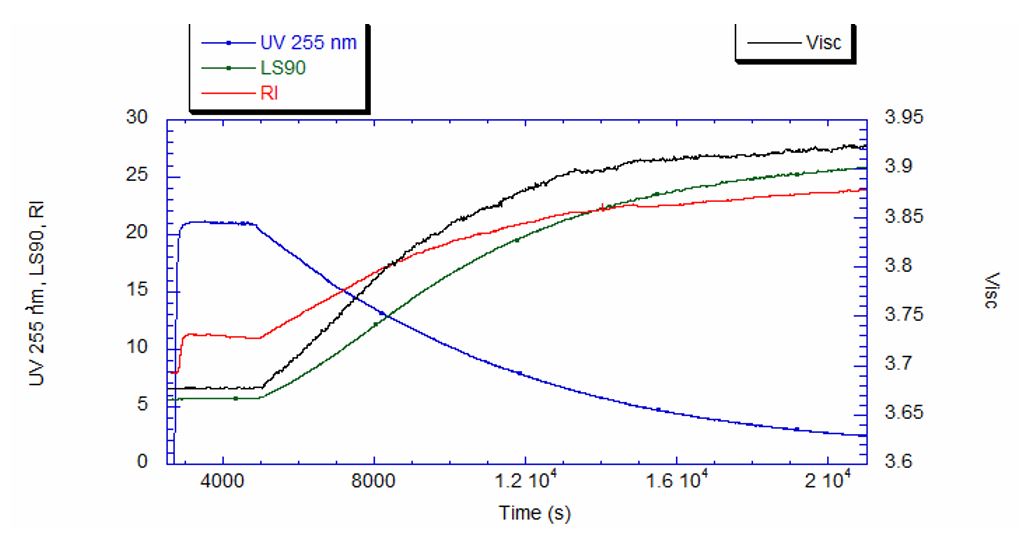
Software installed in the ACOMP uses the raw data, as depicted in Figure 1, to calculate properties of interest like the monomer and polymer concentrations and the weight-average molecular weight (Mw). Figure 2 shows the concentrations of monomer and polymer as measured by the UV detector for both runs. Since these are batch reactions, the polymer concentration increases directly as monomer concentration decreases. With this information, the fractional conversion can be determined, which is displayed in Figure 3.
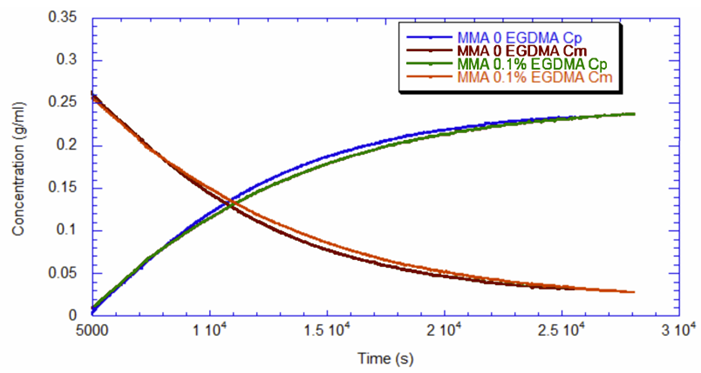
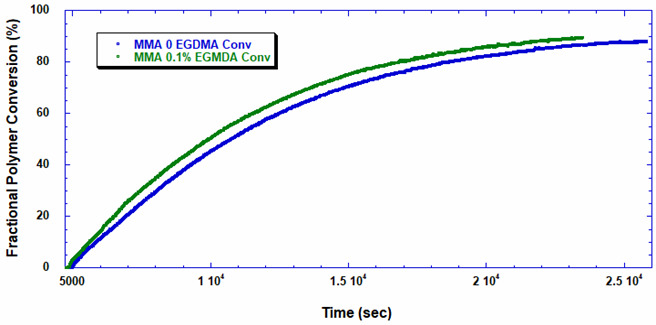
Figure 4 captures the evolution of intrinsic viscosity during both runs of the polymerization. Somewhat counterintuitively, for both reactions the IV goes through a broad plateau and starts to decrease as higher values of polymer concentration are reached (in terms of time, this starts at approximately three hours into the polymerization, which can be seen by comparing to the concentrations in Figure 2). Figure 5 shows that in the absence of crosslinker, Mw also plateaus and turns down at the same time as IV. However, with the crosslinker present, the Mw continues to increase over the next few hours before plateauing at a higher level, which is displayed in Table 2.
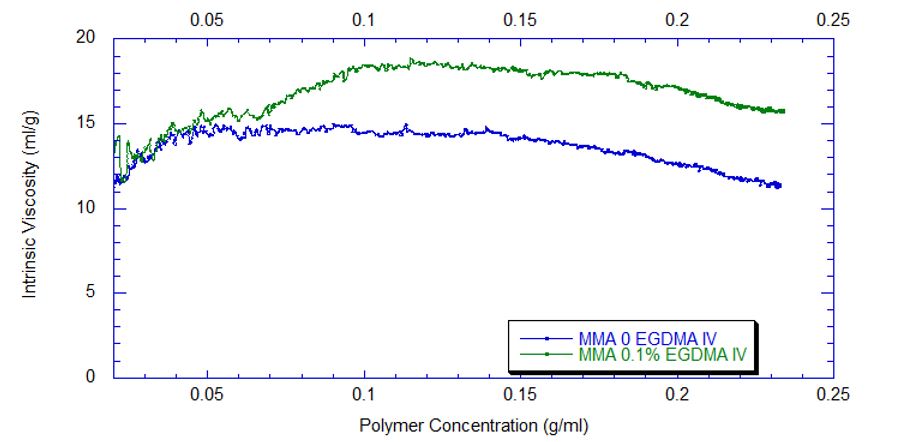
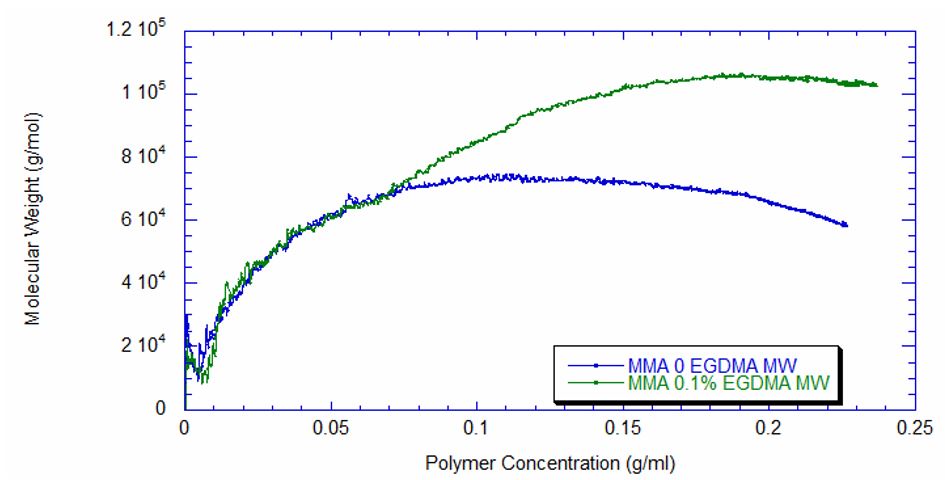
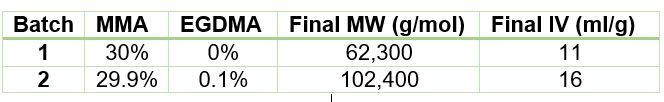
This phenomenon is due to the decrease in monomer concentration so that only molecules of lower molecular weight are formed toward the end of the polymerization. The addition of just 0.1% EGDMA yields a 64% increase in molecular weight and a 45% increase in intrinsic viscosity, suggesting that significant crosslinking has occurred.
Conclusion
ACOMP easily monitors the free radical solution polymerization of polymethylmethacrylate, yielding quantitative results for conversion and other polymer properties. In particular, intrinsic viscosity and molecular weight, important fundamental polymer properties that determine final end-use characteristics, can be measured in real time. The real-time monitoring of these properties enables corrective actions to take place if a recipe is deviating away from the target trajectory. In R&D applications, ACOMP can provide insights into the polymerization and its kinetics that cannot be obtained by post-facto analysis. An example of this is the peak in molecular weight mentioned earlier. These insights can help accelerate R&D by identifying the best pathways to consistently reach the targeted specifications of a polymer product.
In summary, ACOMP is a powerful tool capable of analyzing a wide range of polymerization reactions from R&D and scale-up processes all the way to industrial applications. In addition to ACOMP’s standard detectors, the smart system also includes multiple prescriptive maintenance sensors, qualifying it as self-aware. For industrial or pilot plant scale-up applications, where required, ACOMP is offered as an explosion-proof system.
For Further Reading about ACOMP for Monitoring Acrylates:
Reed, W.F. “Automated Continuous Online Monitoring of Polymerization Reactions (ACOMP) and Related Techniques.” Encyclopedia of Analytical Chemistry, 2013, 1-40.


