Introduction
Understanding the thermal, chemical, and mechanical impacts on protein stability are compulsory for the successful development and delivery of therapeutic proteins. During purification, storage and production processes, proteins are often subjected to stress via high pressure liquid chromatography (HPLC), chemical modification and lyophilization. As a consequence, these processes may result in aggregation, degradation or oxidation of the target protein, rendering it inefficacious. ARGEN™ is the ideal high throughput instrument to understand the effects of thermal, chemical and mechanical perturbations through changes in molecular weight. This allows for rapid vetting of constructs and buffer systems for maximum stability. As a proof of concept (POC), ARGEN was utilized to monitor the thermal stability of human insulin at 50°C, 53.5°C and 57°C, providing data for time to dimer (TD) and time to tetramer (TT) determination.
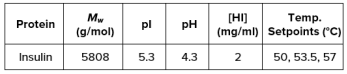
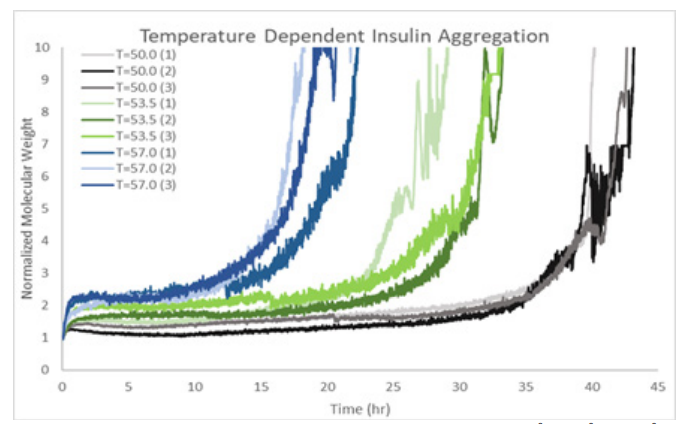
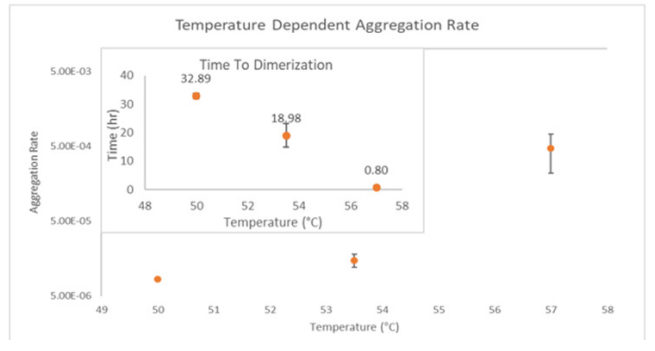
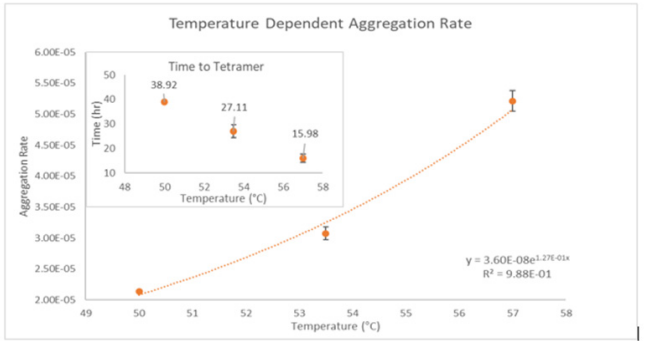
ARGEN™: Smart & Rapid Therapeutic Biopolymer Development ARGEN™ is a high throughput tool for the rapid assessment of the stability and viability of therapeutic proteins, peptides, and biopolymers. The instrument uses a multi-stressor testing platform powered by static light scattering detection and intuitive data processing. These features enable teams to develop biologic formulations up to 16-fold faster. How ARGEN™ Works ARGEN™ utilizes fixed angle (90°), simultaneous multiple sample light scattering (SMSLS) technology which provides rapid, real-time, continuous data collection for characterizing qualitative and quantitative properties of target molecules. The device is equipped with 16 independently controlled sample cells, permitting the user to establish thermal, chemical, and mechanical (stirring) stress parameters on each sample concurrently. This allows for a highly flexible approach to experimental design. ARGEN™ Intuitive Control Software The ARGEN™ control software features an intuitive interface for all aspects of experimental design and independent control of each cell for parallel parameter adjustment and real-time data processing. Human Insulin (Sigma I2643) was dissolved in sodium phosphate buffer at [HI] = 2 mg/mL, pH 4.3. Each sample was analyzed under isothermal conditions as outlined in Table 1 for up to 48 hours. Experimental Parameters Monitoring Thermal Aggregation of Human Insulin Using ARGEN™ Understanding the thermal impacts on protein stability is critical for the successful development of protein based therapeutics. Using Human Insulin as a model, triplicate experiments at each temperature set point (50°C, 53.5°C and 57°C) demonstrated the ability of ARGEN to monitor HI aggregation continuously for up to 48 hours. As seen in Figure 1, at 50°C, the protein remained stable for up to 24 hours, followed by aggregation at time points greater than 24 hours. At 53.5°C, insulin formed a stable dimer for approximately 20 hours subsequent to an exponential increase in aggregation rate at time points greater than 20 hours. At 57°C, insulin formed a stable higher order species with an exponential increase in aggregation rate after 10 hours. Defining Oligomeric State Transitions of Human Insulin Under Thermal Stress Using ARGEN™ Characterizing the transition states of proteins to higher order oligomers and conditions influencing this behavior is key to choosing the ideal construct and developing solution conditions to maximize protein stability. The sensitivity of ARGEN to monitor the kinetics of monomer to dimer (Time to Dimer), dimer to tetramer (Time to Tetramer), and higher order transition states provides key insights into the stability and viability of therapeutic proteins under various thermal, chemical and mechanical stress conditions. Kinetic analysis of the data collected on Human Insulin revealed the time to dimerization (TD) (Figure 2), time to tetramerization (TT) (Figure 3), and aggregation rates (AR) at 50°C, 53.5°C and 57°C set points. As expected, TD and TT decreased with increasing temperature, reflected in increased aggregation rates. TT provided additional insight into the instability of Human Insulin beyond the formation of a stable dimer species as reflected in the exponential aggregation rate (Figure 2). These experiments demonstrate the utility of ARGEN™ to define oligomeric states and transitions of Human Insulin under thermal stress. Time to dimerization and time to tetramerization measurements permitted the characterization of a stable dimer species, as well as insights into the mechanisms and kinetics of higher order oligomer formation. Whereas, SEC allows the characterization of discrete oligomeric species’, integrated data collected using ARGEN identifies the formation of higher order species in real time. Additionally, these experiments can be translated to understand the effects of buffer or solvent conditions, mechanical (stirring) stress and long-term storage (shelf-life) to validate the most robust biotherapeutic constructs.
Experimental Methods
Experimental Results
Conclusion