Introduction
Understanding the thermal, chemical and mechanical stability and physical properties of polymers is essential for biopolymer characterization and development success. The case studies below outline the experimental methodologies and data analysis using ARGEN™ to monitor polymer degradation and to characterize molecular weight as well as the effects of temperature, shear stress and solution conditions on the stability of an uncharacterized organic polymer (PS-A) and Bovine Serum Albumin (BSA). This data provides the pathway to expedite formulation development.
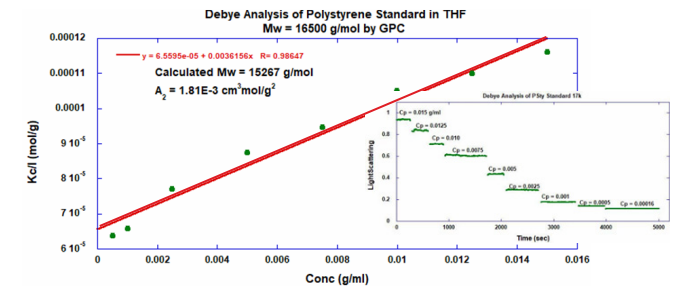
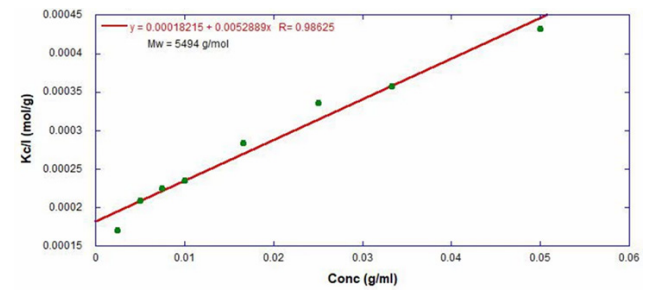
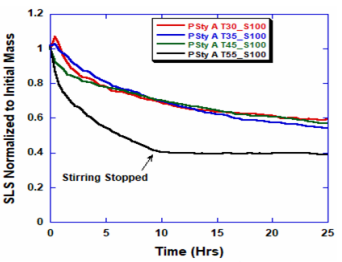
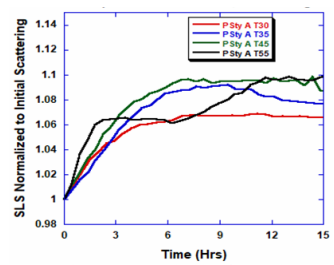
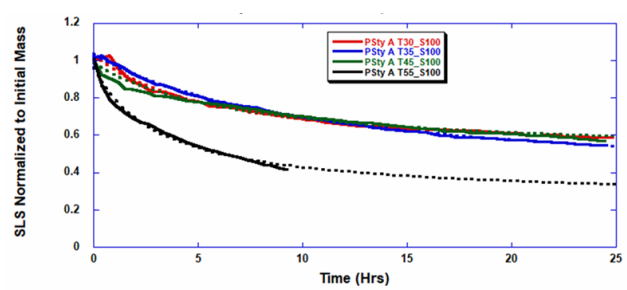
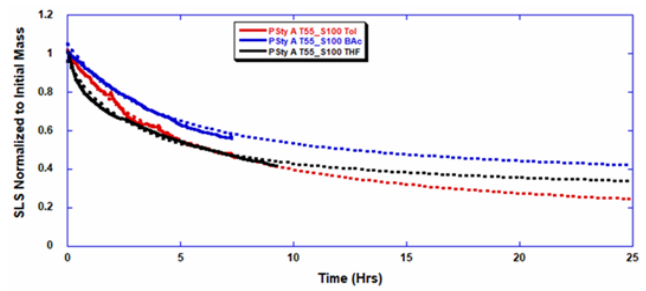
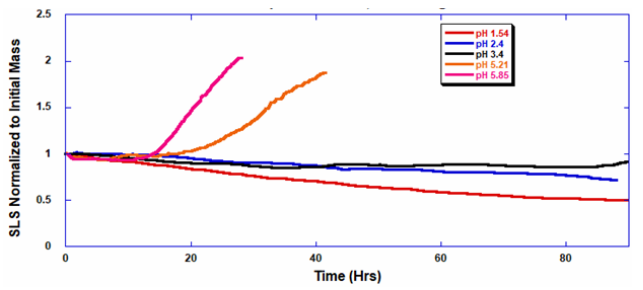
ARGEN™ is a high throughput tool for the rapid assessment of the stability and viability of therapeutic proteins, peptides, and biopolymers. The instrument uses a multi-stressor testing platform powered by static light scattering detection and intuitive data processing. These features enable teams to develop biologic formulations up to 16-fold faster. ARGEN™ utilizes fixed angle (90°), simultaneous multiple sample light scattering (SMSLS) technology which provides rapid, real-time, continuous data collection for characterizing qualitative and quantitative properties of target molecules. The device is equipped with 16 independently controlled sample cells, permitting the user to establish thermal, chemical, and mechanical (stirring) stress parameters on each sample concurrently. This allows for a highly flexible approach to experimental design. The ARGEN™ control software features an intuitive interface for all aspects of experimental design and independent control of each cell for parallel parameter adjustment and real-time data processing. Two Polystyrene (PS) standards, 16,500 g/mol and 185,000 g/mol, and an uncharacterized PS sample (PS-A) were serially diluted with tetrahydrofuran (THF) from 15.0 mg/mL to 0.166 mg/mL, generating an 8-point Debye plot for extrapolation of absolute molecular weight of each sample using the Debye equation: Samples of polystyrene (PS-A) were dissolved in THF at a [PS-A] = 10 mg/ml, placed into cuvettes, and loaded into ARGEN™ sample cells. These samples were then thermally stressed at a temperature range from 30°C – 55°C for 15 hours to monitor degradation. A subsequent experiment was performed with stirring (mechanical stress) at 100 RPM and thermally stressed from 30°C – 55°C for the entirety of the experiment. Finally, three samples of PS-A were dissolved in toluene (Tol), butyl acetate (BAc), or THF and allowed to degrade at 55°C with a stirring rate of 100 RPM for 10 hours. Using Bovine Serum Albumin (BSA) as a model protein for aggregation and degradation, a [BSA] = 2 mg/mL sample was prepared in 50 mM phosphate. The pH of each solution was adjusted, providing a pH range = 1.54 – 5.85 (five data points) with a final [BSA] = 1 mg/ml. Each sample was heated to 37°C for five days using ARGEN’s temperature control feature. Light scattering intensity was recorded for each dilution prior to data collection. Each dilution was analyzed and monitored for at least three minutes to establish a stable baseline signal (Figure 1 – inset). Debye analysis was used to determine the initial molecular weight of each sample, requiring several assumptions. If q2(S2)z << 1 (true for most polymers) and the sample is diluted such that, then the equation can be simplified to: When rearranged for single angle analysis at 90° and solving for weight average molecular weight, the equation becomes: Using this equation and a linear fit of the dilution data (Figure 1), the 16,500 g/mol and 18,500 g/mol standards were calculated to have molecular weights of 15,300 g/mol and 193,000 g/mol, respectively. The unknown PS sample was determined to have a molecular weight of 5,500 g/mol (Figure 2). The polymer degradation curves were normalized to the initial mass of the polymer, and the normalized molecular weight was plotted versus time. As shown in Figure 3, in the absence of mechanical stress (stirring), the polymer was soluble at all temperatures without any observed change in molecular weight and no indication of polymer degradation. In the presence of mechanical and thermal stress, all samples were readily soluble within two minutes, and degradation was observed immediately as indicated by the change in the light scattering signal. Samples subjected to temperatures from 30°C – 45°C displayed a similar degradation profile (Figure 4). At temperatures greater than 55°C, the polymer dissolved rapidly, as displayed in Figure 3. As previously noted, the removal of mechanical stress halts degradation, as evidenced by the change in light scattering intensity. Furthermore, the increase in the polymer degradation rate observed in the temperature range between 45°C to 55°C is indicative of a significant thermodynamic barrier related to the depolymerization of polystyrene (Figure 5). THF and toluene displayed differential effects on PS-A stability and solubility. PS-A dissolved in THF degraded more rapidly, while toluene proved to be a more robust solvent as exhibited by a more steady rate of solvolysis. Based on the double exponential fitting, polystyrene would be less stable in toluene than THF after 10 hours (Figure 6). This phenomena would require further investigation as these organic solvents are not known to participate in the electron transfer required to reduce the polyolefin bonds. Potential impurities in the sample or shear stress may be the root causes of the PS-A degradation. The isoelectric point is the pH (of the solvent or buffer system) at which a biopolymer has no net charge. The isoelectric point of BSA is pH 4.8, and the protein is practically insoluble due to the lack of electrostatic repulsion at a pH 4.8 (buffer system). Upon acidification or basification of the solvent or buffer system containing BSA, the protein becomes either positively (acidic buffer) or negatively (basic buffer) charged, often resulting in an increase in colloidal stability and solubility via electrostatic repulsion. Under thermal stress at pH 5.85 and 5.21 (BSA is negatively charged), the protein aggregates. At a pH ≤ 3.40, acid-catalyzed hydrolysis occurs, resulting in protein fragmentation (creation of peptides) as observed by a decrease in the normalized molecular weight (Figure 7). Between pH 3.40 and pH 1.54, there is a four-fold increase in the protein degradation rate due to an increase in the rate of acid-catalyzed hydrolysis. ARGEN™ was used to successfully determine the molecular weight, elucidate the thermal, mechanical and chemical stability of an uncharacterized polymer, and monitor the solution behavior of bovine serum albumin under several different pH conditions. The molecular weight of PS-A was elucidated by Debye analysis using data generated by ARGEN™. Data collected on thermal, mechanical and chemical stress provided vital information on biopolymer stability. ARGEN™ proved to be a powerful tool with the capability to analyze the stability of both organic polymers and biopolymers in real time and provide the user with the data needed to expedite the formulation development process. ARGEN™: Smart & Rapid Therapeutic Biopolymer Development
How ARGEN™ Works
ARGEN™ Intuitive Control Software
Experimental Methods
Polymer Characterization
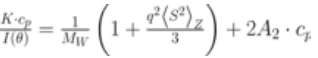
Monitoring Polymer Degradation
Monitoring Biopolymer (Protein) Degradation
Data Interpretation and Analysis
Polymer Characterization
![]()
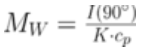
Polymer Degradation
Solvent Effects on Polymer Stability and Solubility
Biopolymer Degradation
Conclusion