Rapid, In Situ, Batch Monitoring of RNA Stability Using ARGEN™: Insights for Expedited RNA Therapeutics Development

Introduction
RNA-based therapeutics are emerging as primary treatment modalities for traditionally “undruggable” targets. Since the approval of the first siRNA drug in 2018 to the expedited development of the COVID mRNA vaccine in 2020, the development of RNA therapies has taken center stage. RNA therapeutics vastly increase the treatment landscape by broadening targets; however, ab initio development presents notable challenges (1). RNA is intrinsically unstable and often requires sequence modification(s) such as 5’ capping, 3’ polyadenylation, incorporation of noncanonical bases, and optimized buffer conditions to ensure metabolic stability, efficacy and prolonged shelf-life. Additionally, the RNA should be engineered for degradation in vivo to decrease half-life and mitigate unwarranted immunological or physiological responses.
Many techniques are employed to study and characterize the stability of engineered RNA for sequence and solution optimization to ensure maximum efficacy and stability during bioprocessing. These include native PAGE, size-exclusion chromatography (SEC), NMR, and PCR. All of these are useful techniques, but they fall short of the batch, in situ, temporal stability monitoring (aggregation and degradation) required to define a comprehensive stability landscape. Higher throughput, batch analysis permits expedited development, getting therapies to market faster.
The ARGEN platform provides consistent and reliable data on the aggregation and degradation propensities of proteins, peptides, and other biopolymers under varying stress conditions (thermal, stirring, buffer). This allows for the rapid optimization of solution conditions to expedite the development of a target protein or peptide for therapeutic use. These applications can be translated to analyze RNA and larger species such as adenoviral vectors and LNP-RNA complexes. This technical note serves as a proof of concept (POC) on the use of ARGEN to monitor the stability of total yeast RNA extract under varied thermal and buffer (pH) conditions.
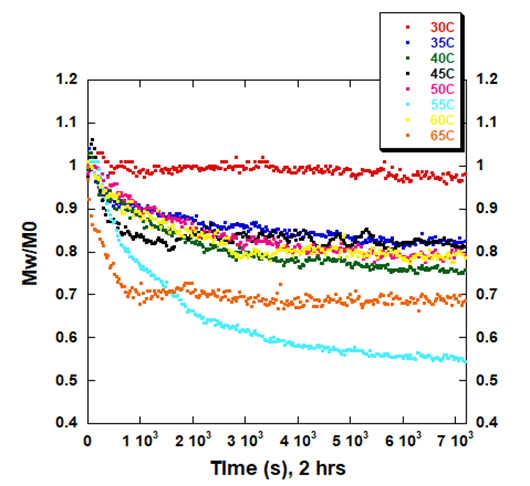
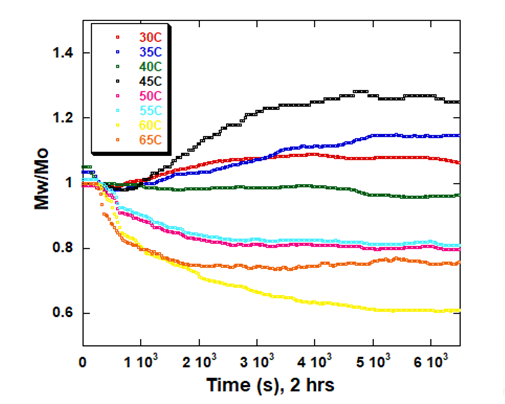
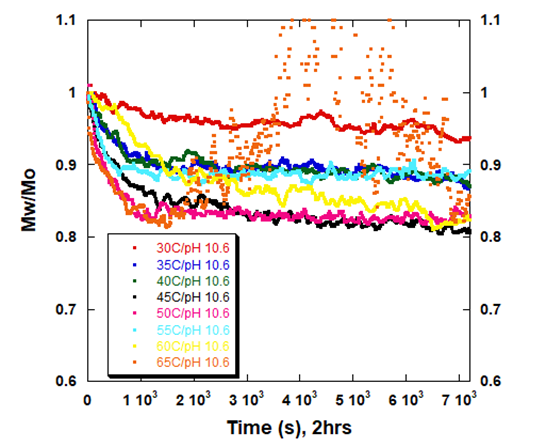
ARGEN is a benchtop lab instrument that enables a smart and rapid approach to therapeutic biopolymer development. The static light scattering platform addresses key challenges in biopharmaceutical R&D. ARGEN utilizes fixed angle (90°), simultaneous multiple sample light scattering (SMSLS) technology which provides rapid, real-time, continuous data collection for characterizing qualitative and quantitative properties of target molecules in situ. ARGEN is equipped with multiple independently controlled sample cells, permitting the user to establish thermal, chemical, and mechanical (stirring) stress parameters on each sample concurrently. This allows for a highly flexible approach to experimental design that accelerates research and development testing efforts from pre-formulation to manufacturing. The ARGEN control software features an intuitive interface for all aspects of experimental design. It also maintains independent control of each cell for parallel parameter adjustment and real-time data processing, providing unprecedented flexibility and creativity in experimentation. The experiments were performed using lyophilized, total yeast RNA extract from Candida utilis purchased from Roche Diagnostics (Ref. 10109223001). Stock samples were prepared at [RNA] = 5 mg/ml in citrate (pH 3.5), phosphate buffered saline (pH 7.4), and carbonate-bicarbonate (pH 10.6) buffers. This was followed by gentle vortexing and sterile filtering using 0.22 µm (pore size) cellulose acetate filters. One milliliter aliquots were loaded into disposable, 1 cm pathlength, plastic (methacrylate) cuvettes and capped for right angle light scattering experiments. Each experiment analyzed eight samples in parallel within an (iso)thermal range of 30°C-65°C in citrate (pH 3.5), phosphate buffered saline (pH 7.4), and carbonate buffer (pH 10.6) buffers for a duration of two hours to rapidly monitor thermal stability. Each sample cell was equilibrated to the temperature set point prior to experimentation. All supplies, vessels and filters were sterile and RNase free. Data was collected using ARGEN™, a higher throughput, batch processing static light scattering (SLS) device (λ=600 nm, 90° detection) developed by Yokogawa Fluence Analytics and patented on the principle of simultaneous multiple sample light scattering (SMSLS). The instrument is equipped with eight autonomous sample cells permitting independent experimental parameter (temperature and stirring rate) adjustment. Additionally, each sample cell is engineered with a dedicated, fixed optical detector train to mitigate measurement error(s). Absolute scattering intensities derived from the RNA thermal stability experiments were normalized (to 1 at t = 0s) and represent the molar mass (weighted average) of the unperturbed, native sample (Mo) at t = 0s. Mw(t) is the molar mass (weighted average) of the sample as represented by temporally resolved changes (x-axis) in light scattering intensity. The dimensionless quantity Mw/Mo represents the molecular weight of the aggregate mass (Mw) of all species (molecular weight of all RNA species in the sample) with respect to the molar mass of the unperturbed, native state (Mo) of the sample and is displayed as ‘Mw/Mo’ (y-axis). For reference, a normalized intensity equal to two represents (Mw/Mo = 2) means the RNA has transitioned to twice the molar mass (average) of the native species (aggregated or unaggregated). A deviation below Mw/Mo = 1 may represent an increase in solubility or degradation to a lower order species. The exquisite sensitivity of static light scattering permits the rapid characterization of temporal changes in average molecular weight in situ under various global and local stressors, enabling a rapid optimization of conditions for maximum stability. As a proof of concept (POC) for RNA applications, ARGEN was utilized to characterize the thermal stability and solution behavior of a total RNA extract from Candida utilis under three different buffer systems (pH) within an (iso)thermal range of 30°C-65°C. The RNA was prepared in citrate pH 3.5, phosphate buffered saline (PBS) pH 7.4, and carbonate-bicarbonate pH 10.6 buffers to provide a stability landscape at these temperatures and pH conditions using ARGEN. Observed changes in weight average molecular weight (Mw(t)/Mo) over time indicated significant differences in thermal stability, solubility and degradation (hydrolysis) propensity as related to buffer conditions (pH). Increases in Mw/Mo indicated aggregation, while decreases in Mw/Mo indicated either an increase in solubility (disassembly of loosely associated aggregates) or hydrolysis. Due to the higher throughput (eight sample cells) and batch processing capacity of ARGEN, all three datasets (Figures 1, 2 and 3) were each generated in two hours, permitting expedited vetting of solution conditions required for further investigation and formula optimization. The RNA was prepared in citrate buffer (pH 3.5) and thermally stressed from 30°C-65°C to monitor thermal stability and acid-catalyzed hydrolysis. Interestingly, the RNA was most stable at 30°C, as indicated by minimal changes in Mw/Mo (Figure 1). As observed, Mw/Mo decreased slightly for ~500s followed by minimal changes in Mw/Mo for the duration of the experiment. The initial decrease in weight average molar mass may indicate a disassembly of loosely associated aggregates translating to an increase in solubility. Since static light scattering is not suitable to characterize heterogeneity, it is often necessary to couple it with size-exclusion chromatography (SEC) or dynamic light scattering (DLS) experiments to confirm the polydispersity of the sample. Significant decreases in Mw/Mo were observed at temperatures >30°C. Again, this may be indicative of an increase in solubility or acid-catalyzed hydrolysis (degradation). At 55°C, the weight average molar mass average decreased by ~50% over two hours. The RNA behaved similarly from 35°C-45°C apart from 60°C where the molar mass average was grouped with these samples. Analysis at 65°C showed a rapid decrease in Mw/Mo, followed by a slight increase up to 2000s. It then stabilized to ~70% of Mo (average molar mass at t = 0s). Figure 1: Mw/M0 vs. time (s) for total RNA (Candida utilis) in citrate buffer (pH 3.5) from 30°C-65°C RNA is acidic and most stable from pH 4-5. However, therapeutic RNA should be analyzed in situ and in real-time under physiological conditions for sequence and buffer optimization to ensure stability and efficacy in vivo. Monitoring aggregation and degradation propensities in situ and in real-time allows for a rapid pivot on formulation design and a streamlined development. For these experiments, eight samples of the crude yeast RNA were prepared in phosphate buffered saline at pH 7.4. They were analyzed in parallel from 30°C-65°C for two hours to determine disparities in aggregation or degradation properties within this thermal range (Figure 2). At 40°C, the RNA remained stable with diminutive changes in Mw/Mo throughout the duration of the two hour experiment. Mw/Mo changes at 30°C, 35°C and 45°C indicate aggregation and the formation of higher order species, likely mediated by intermolecular base pairing, due to dismantling of secondary and tertiary structure. The melting point (Tm) of a typical RNA is ~50°C-60°C. Temporal changes in Mw/Mo at thermal set points in this experiment from 50°C-60°C seem to be reflective of this property. However, it should be noted that crude RNA is composed of many different species. Thus, it is impossible to predict intra- and intermolecular base pairing or secondary and tertiary structural element contributions to thermal stability. Therefore, the initial, rapid decrease in Mw/Mo at 65°C followed by an increase in Mw/Mo from 2000s to 6500s may indicate denaturing of the RNA, followed by intermolecular base pairing and the formation of higher order species. Characterizing this behavior becomes more accurate and precise when these techniques are employed for the analysis of an enriched, single species. Figure 2: Mw/M0 vs. time (s) for total RNA (Candida utilis) in phosphate buffered saline (pH 7.4) from 30°C-65°C Bulk yeast RNA was prepared in carbonate-bicarbonate buffer (pH 10.6) to characterize the effects of high pH under thermal stress and demonstrate the utility of ARGEN for in situ and batch (eight samples) monitoring of base-catalyzed degradation. The RNA remained relatively stable at 30°C with a ~5% change in weight average molar mass (Mw/Mo) for the two hour duration of the experiment (Figure 3). As temperature increased from 35°C-65°C, Mw/Mo decreased rapidly, which may represent base-catalyzed hydrolysis or an increase in solubility. Scattering profiles from experiments performed at 35°C, 40°C and 55°C represented a reduction in average molar mass change by ~10% in contrast to a ~15% reduction at 50°C. Interestingly, the weight average molar mass decreased by ~20% at 45°C which likely indicates the ideal temperature for maximum solubility. At 65°C, RNA degradation was followed by aggregation, as represented by a rapid increase in scattering intensity (orange trace) at 1500s (25 minutes). Figure 3: Mw/M0 vs. time (s) for total RNA (Candida utilis) in carbonate-bicarbonate buffer (pH 10.6) from 30°C-65°C RNA-based therapies are becoming standard and primary treatment modalities for many disease conditions. However, the development of these therapies presents notable challenges. RNA is intrinsically unstable, which requires precise sequence engineering and optimized stabilizing solution conditions for effective storage and efficacy. Defining quantitative and qualitative properties of RNA and RNA-LNP complexes in an expedited manner is often the threshold for development success. The experiments presented in this technical note serve as a POC for higher throughput, in situ, and temporal analysis of RNA under thermal stress in different buffer systems (pH) using ARGEN. Total RNA extract from Candida utilis was used as a cost-effective resource for the POC. However, more precision and accuracy can be obtained using enriched or purified samples. ARGEN is a novel static light scattering instrument patented on the principle of simultaneous multiple sample light scattering which permits the user to monitor solution behavior of up to eight samples in parallel. The ability to monitor changes in weight average molecular weight over time enables rapid formulation development and optimization. In addition to monitoring the effects of temperature and solution conditions on stability as demonstrated above, applications can be extended to monitoring the temporal stability of RNA down to 3°C for shelf-life determination. Each sample cell is equipped with a magnetic stepper motor (coupled to a stir bar) which allows for modeling the effects of stirring stress encountered during bioprocessing. ARGEN™: Accelerating Biotherapeutic Formulation Development
How ARGEN™ Works
ARGEN™ Intuitive Control Software
Materials and Methods
Materials
Methods
Results
Monitoring Acid-catalyzed Hydrolysis of RNA
Monitoring Thermal Stability and Solubility of RNA
Monitoring Base-catalyzed Hydrolysis of RNA in Carbonate-bicarbonate Buffer
Conclusion
References