Coupling SLS and DLS Data to Characterize Solution Behavior of rAAVs:
A Case for Successful Development and Production

Introduction
Recombinant adeno-associated vectors (rAAVs) have proven to be viable treatment options for the successful management of many single gene disease states. Despite their therapeutic potential, the development of efficacious rAAV vectors is challenging and requires a comprehensive understanding of their stability landscape under a range of thermal, chemical, storage, and mechanical (stirring) stress conditions. Quantitative and qualitative stability data are essential to the design of stable formulations which can be translated to successful purification, processing, manufacturing, and storage conditions.
The data presented in this paper is generated from static light scattering (SLS) and dynamic light scattering (DLS) experiments that monitored the solution behavior of empty (without DNA) and full (with DNA) rAAV capsids. The comparison and superimposition of SLS and DLS scattering profiles provides insights into the differential colloidal stability of the full and empty species, as well as a dynamic quantification of genome ejection. (1)
ARGEN is a benchtop lab instrument that enables a smart and rapid approach to therapeutic biopolymer development. The static light scattering platform addresses key challenges in biopharmaceutical R&D. ARGEN utilizes fixed angle (90°), simultaneous multiple sample light scattering (SMSLS) technology which provides rapid, real-time, continuous data collection for characterizing qualitative and quantitative properties of target molecules in situ. ARGEN is equipped with multiple independently controlled sample cells, permitting the user to establish thermal, chemical, and mechanical (stirring) stress parameters on each sample concurrently. This allows for a highly flexible approach to experimental design that accelerates research and development testing efforts from pre-formulation to manufacturing. The ARGEN control software features an intuitive interface for all aspects of experimental design. It also maintains independent control of each cell for parallel parameter adjustment and real-time data processing, providing unprecedented flexibility and creativity in experimentation. rAAVs were produced by triple transfection in HEK-293 cells and purified from crude cell lysate by ion-exchange chromatography. Empty (without DNA) and full capsids (with 4.7 kb, ssDNA) were concentrated and dialyzed into 20mM sodium phosphate, 180 mM NaCl at pH 7.3. Prior to the experiments, full and empty capsid samples were filtered using a 0.22 µm cellulose acetate filter. Data was collected using ARGEN, an SLS device (λ=660 nm, 90° detection) developed by Yokogawa Fluence Analytics and patented on the principle of simultaneous multiple sample light scattering (SMSLS). The instrument is equipped with eight autonomous sample cells, permitting independent experimental parameter (temperature and stirring rate) adjustments. Additionally, each sample cell is engineered with its own optical detector train. Absolute molecular weight (Mw) determination of full and empty capsids was achieved using the Zimm approximation. Mw(t) is the molecular weight (average) of the capsid (full or empty) as represented by time resolved light scattering intensity. Mo is the initial molecular weight of the capsid (full or empty) prior to aggregation at t = 0. Mw(t)/Mo represents the temporal change in average molecular weight (average) with respect to the native state of the capsid(s). All experiments were performed in 1 cm pathlength, disposable cuvettes with 1 ml sample volumes. DLS is a robust technique which permits the determination of hydrodynamic diameter (dH) as well as the polydispersity index (PI) of particles or molecules in solution. The principle of DLS is based solely on Brownian (random diffusion) motion and elastic collisions of dispersed particles. Simply, smaller particles diffuse more rapidly (and collide more often) than larger particles, and this relationship is defined by the autocorrelation function to determine the translational diffusion coefficient (Do, units of m2/s). Finally, the Stokes-Einstein equation (below) is used to determine the hydrodynamic diameter, dH, as related to Do. For DLS, particles are assumed to be monodisperse and uniformly dense spheres so this may introduce error upon comparison with other techniques depending on the polydispersity and shape deviation. The PI is obtained from the standard deviation of Do, and reconciled to the width of the size distribution. Stokes-Einstein Equation: The ARGEN platform is equipped with eight independently controlled and operated sample cells engineered with optics for RALS detection. RALS is complementary to DLS, as it permits the detection of temporal changes in molar mass (weighted average, Mw/Mo) and is exquisitely sensitive to oligomeric state transitions (aggregation) and degradation. For these experiments, initial scattering intensities were normalized to 1 and represent the molecular weight (average) of the unperturbed, native sample (Mo) at t = 0s. Mw(t) is the molecular weight (average) of the sample as represented by temporally resolved (x-axis) light scattering intensity changes. Mw(t)/Mo represents the weight of the aggregate mass (Mw(t)) with respect to the unperturbed, native state (Mo) and is displayed as “SLS Mw/Mo” (y-axis). An increase in Mw/Mo indicates an increase in weight average molar mass, while a decrease in Mw/Mo is indicative of a decrease in weight average molar mass. A comparison of RALS and DLS data confirms differences in solution behavior between empty and full capsids under thermal stress (44°C). The superimposition of RALS and DLS data exhibit similar qualitative profiles for empty capsids (Figure 1, Panel A) with increases in Mw/Mo (SLS) and <dH>z,ap (DLS, “DLS apparent Diameter”) temporally. The increase in diameter for empty capsids is ~7-fold (DLS) which equates to ~50 aggregated capsids (SLS, weighted average). Conversely, data from full capsids (60%) shows a decrease in Mw/Mo (SLS) with an increase in <dH>z,ap (Figure 1, Panel B) over 7 hours (~25000s). This is consistent with genome ejection from the full capsids as the 2.4 MDa ssDNA would have a radius of gyration (Rg) of 36 nm, while the full capsid has a Rg = 22 nm. Table 1 provides a comparison summary of SLS and DLS data. Table 1: Summary of SLS and DLS data collected from full and empty capsids Defining a comprehensive stability landscape under local and global stress conditions is compulsory for rAAV development success. Coupling analytical techniques such as SLS and DLS provides the increased resolution needed for formula optimization. As shown, the superimposition of SLS and DLS data provided conclusive results on the differential solution stability of empty and full capsids under thermal stress (44°C) as well as the release of DNA from the capsid. The unique ability of ARGEN to monitor aggregation and degradation propensities in situ and in real time under stirring stress and at low temperature (3°C-8°C) for up to eight samples in parallel permits a higher throughput stability analysis under bioprocessing and storage conditions. Additionally, these applications can be extended to all classes of biologics, including larger LNP-RNA complexes. ARGEN™: Accelerating Biotherapeutic Formulation Development
How ARGEN™ Works
ARGEN™ Intuitive Control Software
Materials and Methods
Materials
Methods
Comparing SLS and DLS Data: Evidence for Genome Ejection from rAAVs
Dynamic Light Scattering (DLS)
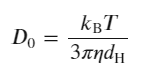
Right Angle Light Scattering (RALS): SLS at 90°
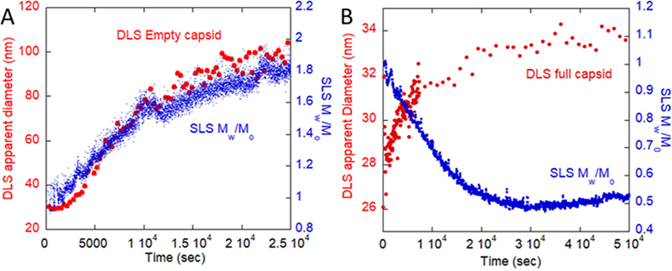
Empty Capsids
Full Capsids (60%)
Mw/Mo (SLS)
Increase
Decrease (Ejected DNA + capsid)
<dH>z,app (DLS)
Increase
Increase (extension of DNA upon release from capsid)
Conclusion
References


