DNA Released by Adeno Associated Virus Strongly Alters Capsid Aggregation Kinetics in a Physiological Solution
The academic research partner of Yokogawa Fluence Analytics, Tulane PolyRMC, partnered with Spark Therapeutics on a novel peer-reviewed article in Biomacromolecules. The publication highlights the degradation of adeno-associated virus (AAV) under moderate thermal stress (30°C−53°C) and under physiological conditions (pH7.2, 180 mM NaCl). While adeno-associated virus is a leading vector for gene therapy, significant gaps remain in understanding AAV degradation propensity and solution stability.
For these experiments, viral particles of varying fractions of encapsulated DNA were incubated between 30°C and 60°C. Temporal changes in molecular weight were measured by ARGEN, a high throughput static angle light scattering platform that helps biopharmaceutical teams worldwide rapidly understand biopolymer stability in real time under thermal, mechanical, and chemical stress.
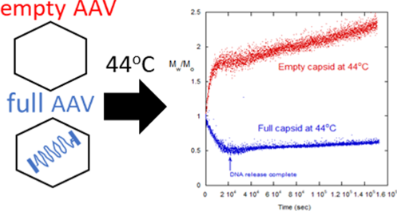
Generally, full vectors demonstrated a rapid decrease in molecular weight, which correlated with the release of capsid DNA followed by slow aggregation, comparatively. In contrast, empty vectors underwent rapid colloid-type aggregation. Mixtures of full and empty capsids showed a significant decrease in initial aggregation that cannot be explained by a linear superposition of empty and full degradation scattering signatures. This indicates interactions between capsid proteins and ejected DNA that influenced aggregation mechanisms.
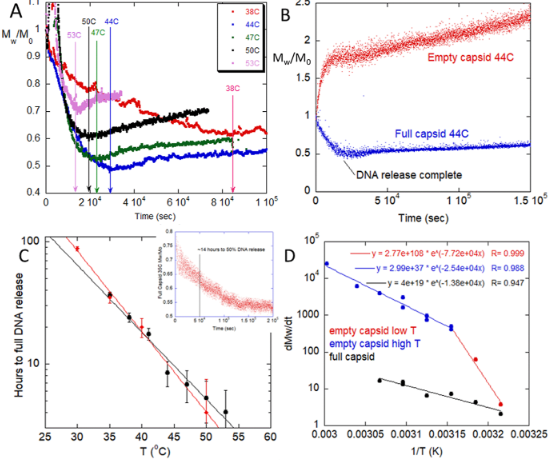
Experiments probing the empty/full ratio and digestion of released DNA showed that DNA is a major component in influencing the capsid degradation pathway. This suggests that colloidal and/or structural instability from the capsid proteins is inducing capsid aggregation. This phenomena also intimates that the ejected DNA alters the degradation pathway of capsids and capsid fragments.
The results of this work, which could not have been attained without ARGEN, provide key implications toward the storage of AAV pharmaceuticals. The degradation propensities of empty versus full AAVs suggests that empty content and DNA cargo will influence degradant species and degradant formation rates during drug product storage. Therefore, AAV degradation and storage lifetime may be dependent on not just capsid serotype, storage temperature, and storage solution matrix but also DNA size and abundance of product variants, such as empty capsids.

