The Effect of Protein Concentration on Aggregation Propensity Under Stirring Stress: Implications for Manufacturing

Introduction
The successful development and manufacture of protein-based therapeutics requires a comprehensive understanding of protein concentration, solution behavior, and colloidal stability under a myriad of local and global stressors. Characterizing responses to stirring stress using benchtop instrumentation provides higher throughput capabilities for optimizing buffer systems and concentrations.
This case study was performed using bovine serum albumin (BSA) as a model protein system for demonstrating the utility of ARGEN™ to characterize aggregation propensity under stirring stress, as a function of protein concentration in situ and in real-time. This data permits the user to optimize protein concentration and solution conditions during stirring phases of manufacturing to mitigate or limit the formation of higher order species and subsequently concentrate as desired.
ARGEN is amenable to in situ and real-time analysis of up to eight samples in parallel under stirring or thermal stress, making it ideal for rapid grid screening and design of experiments (DOE). Elucidating the impacts of stirring and shear stress on biologics during processing and manufacturing is key to rapid and successful development and provides the final link in getting a treatment to market.
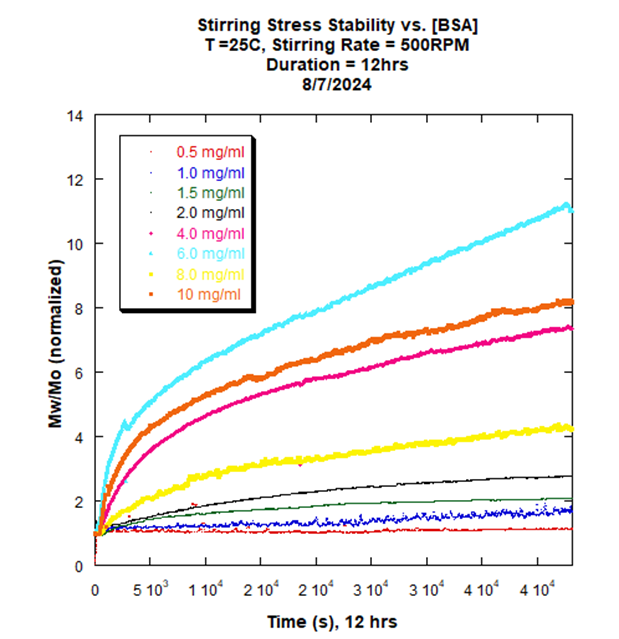
ARGEN is a benchtop lab instrument that enables a smart and rapid approach to therapeutic biopolymer development. The static light scattering platform addresses key challenges in biopharmaceutical R&D. ARGEN utilizes fixed angle (90°), simultaneous multiple sample light scattering (SMSLS) technology which provides rapid, real-time, continuous data collection for characterizing qualitative and quantitative properties of target molecules in situ. ARGEN is equipped with multiple independently controlled sample cells, permitting the user to establish thermal, chemical, and mechanical (stirring) stress parameters on each sample concurrently. This allows for a highly flexible approach to experimental design that accelerates research and development testing efforts from pre-formulation to manufacturing. The ARGEN control software features an intuitive interface for all aspects of experimental design. It also maintains independent control of each cell for parallel parameter adjustment and real-time data processing, providing unprecedented flexibility and creativity in experimentation. A stock solution of [BSA] = 10 mg/ml was prepared in 50 mM phosphate buffered saline (PBS), pH 7.4 and subsequently serially diluted from [BSA] = 0.5 mg/ml-10 mg/ml (7.5 µM-150 µM). Solutions were subsequently filter sterilized using 0.22 µm cellulose acetate filters, and 1 ml aliquots were loaded into cuvettes prior to static light scattering experiments. Contact stirring experiments were performed using ARGEN, a fixed-angle (90°) static light scattering device (λ=600 nm) developed by Yokogawa Fluence Analytics and patented on the principle of simultaneous multiple sample light scattering (SMSLS). The instrument is equipped with eight autonomous sample cells which permit independent experimental parameter (temperature and stirring speed) adjustment on each sample. Each sample cell is equipped with its own laser and optical detection system. There are no moving parts or components in the optical path, so alignment of the light sources (laser) and detector fibers are conserved to mitigate scattering intensity measurement errors. Furthermore, each sample cell is equipped with a magnetic stepper motor which couples to the micro stir bar at the bottom of the cuvette (contact surface) to simulate perturbations experienced during mixing and bioprocessing. The stirring rate (up to 2000 RPM) is manually controlled for precision via ARGEN’s intuitive control software. All stirring experiments were performed using optical glass cuvettes (1 cm pathlength, Spectrocell RF-0010-T). Magnetic stir bars were poly-(tetrafluoroethylene)-coated with dimensions of 2 mm × 5 mm. Initial scattering intensities were normalized to 1 and represent the molecular weight (average) of the unperturbed, native protein sample (Mo) at t = 0. Mw(t) is the molecular weight (average) of the protein as represented by time resolved (x-axis) light scattering intensity changes. Mw(t)/Mo represents the ratio of aggregate mass (Mw(t)) with respect to the unperturbed, native state (Mo) of the protein at time (t) and is displayed as “Mw/Mo (normalized)” (y-axis). For example, a normalized intensity equal to two represents, Mw(t)/Mo = 2. If the protein or peptide sample is monomeric prior to starting the experiment, then Mw(t)/Mo = 2 means the protein has transitioned to twice the molecular weight (average) of the lowest order species at t = 0. All stirring experiments were performed using 1 cm pathlength glass cuvettes manufactured by Spectrocell and SP Bel-ART spinbar teflon micro (flea) stir bars (8mm x 3mm). Discovering and defining optimal protein concentration, solution conditions, and methods for the successful manufacture of biologics can be challenging. The ability to characterize aggregation and degradation propensities in response to stressors encountered during bioprocessing in situ and in real-time, prior to up-scaled production, can save valuable time and resources. Monitoring subvisible oligomeric state transitions in response to stirring (shear) stress experienced during manufacturing permits early detection of instability to expedite formula optimization and streamline development. For this case study, BSA was serially diluted from a stock solution (10 mg/ml) from 0.5 mg/ml to 10 mg/ml in 0.5 mg/ml increments (eight samples total) to determine the effect of aggregation propensity and temporal progression to higher order species under stirring parameters of 500 RPM for 12 hours. Interestingly, BSA remained stable at 0.5 mg/ml with aggregation propensity slightly increasing up to 2.0 mg/ml with the highest order species representing a dimer (molar mass average), Mw(t)/Mo = ~2 (Figure 1). At concentrations ranging from 4.0 mg/ml to 10 mg/ml, aggregation proceeded in a quasi-stochastic manner with the highest order aggregate forming at 6.0 mg/ml, as indicated by a Mw(t)/Mo = ~11mer (molar mass average). It should be noted that higher order aggregate size determinations for BSA are only estimates, as they are not likely Rayleigh scatterers and require analysis at multiple angles for a precision assessment. This behavior is counterintuitive and may be reconciled to concentration-related viscosity differences and stir bar instability at higher concentrations. It may also be an intrinsic property of BSA. Further investigation is required. Figure 1: Normalized scattering intensity vs. time(s) for [BSA] = 0.5 mg/ml to 10 mg/ml The ability to characterize the effects of stirring stress on biologics in situ and in real-time is compulsory for successful development and production. For this case study, ARGEN was used to determine the effect of protein concentration on the aggregation propensity under stirring stress using bovine serum albumin (BSA) in phosphate buffered saline, pH 7.4. At concentrations ≦2.0 mg/ml, aggregate sizes increased with increasing [BSA], as indicated by changes in Mw(t)/Mo. At higher concentrations (4 mg/ml to 10 mg/ml), aggregation proceeded in a quasi-stochastic manner with the largest aggregates forming at 6 mg/ml. Characterizing this solution behavior enables the optimization of protein concentration and solution conditions required to mitigate instability and aggregation during manufacturing and processing. ARGEN™: Accelerating Biotherapeutic Formulation Development
How ARGEN™ Works
ARGEN™ Intuitive Control Software
Materials
Experimental Methods
Contact Stirring Experiments
Discussion
Conclusion


